A New Antibiotic from Nature + Live Science Chat this Friday!
Scientists have discovered a new broad-spectrum "lasso peptide" antibiotic.

Antibiotic resistance is one of the greatest threats to global health, contributing to more than 4.5 million deaths in 2019 alone. As bacteria evolve defenses against our current arsenal of drugs, especially those targeting Gram-negative pathogens deemed critical threats by the World Health Organization, the need for new antibiotics becomes increasingly urgent. Historically, many effective antibiotics—such as penicillins, cephalosporins, vancomycin, and colistin—have been derived from microbes. However, the growing prevalence of resistance mechanisms, particularly those that neutralize antibiotics targeting common ribosomal sites, demands innovation in how and where we attack bacterial cells.
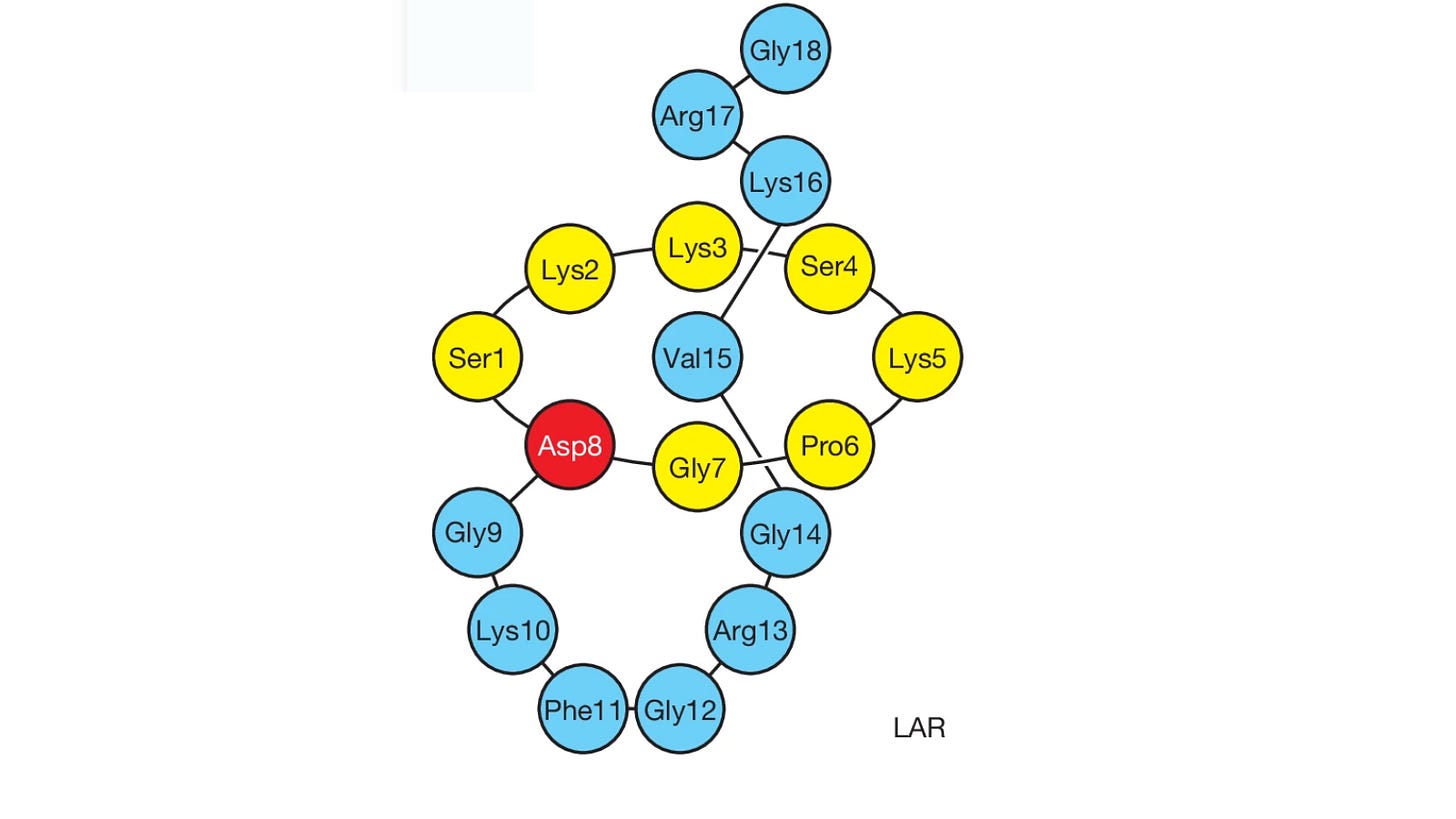
One promising frontier in antibiotic discovery lies in a class of natural compounds called lasso peptides. These are ribosomally synthesized and post-translationally modified peptides (RiPPs), meaning they’re initially made by the cell’s ribosome and then structurally customized by enzymes. Lasso peptides are named for their distinctive lariat-like shape: a molecular "knot" where the tail of the peptide threads through a ring formed by an internal chemical bond. This structure gives them exceptional stability and can enable potent antimicrobial activity. In a recent breakthrough, scientists discovered a new lasso peptide, lariocidin (LAR), which targets the bacterial ribosome at a previously untapped site. LAR not only halts protein synthesis in bacteria but also causes translation errors, showing broad activity against both Gram-positive and Gram-negative pathogens—including effectiveness in animal infection models.
Live Science Chat with Dr. Wright on Friday
Join me this Friday for a live streamed interview with the senior author of the study, Dr. Gerry Wright! Dr. Wright is a professor in the Department of Biochemistry and Biomedical Sciences at McMaster University. Gerry established the Institute of Infectious Disease Research (IIDR) and Canada's Global Nexus for Pandemics and Biological Threats at McMaster University, and served as Director for many years, steering both institutions through significant growth and transformation.
You’ll receive a notification by email when the Science Chat stream goes live. You can also access the stream directly on the Substack app when it goes live.
Date: This Friday, April 4th
Time: 11 AM Pacific Time / 2 PM Eastern Time (USA)
Can’t make it? Don’t worry, I will record the session and post it so you can watch at a later date if interested.
To prepare for the interview, we’d love to get your questions in advance! You can also post them in the chat function during the livestream, but advance questions will get priority. Post your questions here in the comments section.
Now, let me give you some more context on the study and the major findings. You can find the final version of the article in Nature (see link to paper below, behind a paywall), or you can read the open-access pre-print version at Research Square.
Jangra M, Travin DY, Aleksandrova EV, Kaur M, Darwish L, Koteva K, Klepacki D, Wang W, Tiffany M, Sokaribo A, Coombes BK, Vázquez-Laslop N, Polikanov YS, Mankin AS, Wright GD. A broad-spectrum lasso peptide antibiotic targeting the bacterial ribosome. Nature. 2025 Mar 26. doi: 10.1038/s41586-025-08723-7.
Study Methods
To discover the new antibiotic lariocidin (LAR), scientists turned to an unexpected source—soil from Hamilton, Canada. They gave soil bacteria plenty of time to grow, even letting some plates sit for up to a year to allow slow-growing microbes a chance to emerge. From this rich microbial mix, the team isolated 80 different bacterial strains and tested them to see if any could kill harmful bacteria. One promising strain, named Paenibacillus M2, stood out by effectively fighting off both E. coli and Acinetobacter baumannii, two tough-to-treat pathogens. The researchers then fine-tuned the bacteria’s growing conditions to produce more of the active compound and used a series of purification steps to isolate LAR in its pure form. They analyzed its structure using high-tech tools like mass spectrometry and NMR—methods that let scientists "see" the molecular makeup of the compound.
To better understand how LAR is made and how it works, the team sequenced the bacterium’s DNA and identified the set of genes responsible for producing LAR. They then transferred these genes into a different, easier-to-handle bacterial species to confirm that the same compound could be made outside of the original soil bacterium. To test how well LAR worked, they measured how much was needed to stop bacterial growth, examined whether it harmed human cells (it didn’t), and ran experiments to uncover how it attacks bacteria. Finally, the antibiotic was tested in mice and in a model that mimics infection in human blood.
Major Findings
What makes this new discovery, LAR, stand out is how it kills bacteria—it shuts down their ability to make proteins by targeting the ribosome, a molecular machine essential for life. Unlike most antibiotics, LAR binds to a completely new spot on the ribosome, which makes it harder for bacteria to develop resistance. Even more exciting, LAR was effective in fighting drug-resistant infections in animals, making it a promising lead in the search for new antibiotics.
Key points:
Found in soil: LAR was discovered after researchers let soil bacteria grow for a full year, allowing them to find slow-growing microbes often missed in standard searches.
Unique structure: LAR has a special looped shape—like a tiny lasso—that makes it extremely stable. One version, LAR-B, even has a second loop, which is completely new among known antibiotics.
Novel mode of action: LAR blocks bacteria from making proteins by jamming up the ribosome in a way that no other antibiotic does. It also causes the bacteria to make errors in protein building, which helps kill them.
Effective against tough microbes: LAR works against both Gram-positive and Gram-negative bacteria, including dangerous drug-resistant strains like Acinetobacter baumannii.
Hard for bacteria to resist: Because LAR targets a new site on the ribosome, bacteria aren’t able to easily evolve resistance the way they can with older antibiotics.
Could be safe for humans: LAR showed no harmful effects on human cells or red blood cells, and didn’t affect helpful gut bacteria or fungi.
Worked in mice: In infected mice, LAR dramatically reduced the number of bacteria and improved survival, even against a deadly drug-resistant infection.
Room to grow: Similar LAR-like compounds may exist in other bacteria, offering future opportunities to tweak and improve this class of antibiotics even further
The Takeaway
Antibiotic resistance continues to rise, and there is an urgent need to fuel the drug development pipeline with new discoveries like this one. The journey from finding medicines in nature to developing them into treatments for people is long and challenging, and I’m grateful for colleagues like Dr. Wright and his team who are leading the charge in this search for new solutions. I hope you’ll join us this Friday for our live science chat about this exciting discovery!
Yours in health, Dr. Quave
Cassandra L. Quave, Ph.D. is a Guggenheim Fellow, CNN Champion for Change, Fellow of the National Academy of Inventors, recipient of The National Academies Award for Excellence in Science Communication, and award-winning author of The Plant Hunter. Her day job is as professor and herbarium director at Emory University School of Medicine, where she leads a group of research scientists studying medicinal plants to find new life-saving drugs from nature. She hosts the Foodie Pharmacology podcast and writes the Nature’s Pharmacy newsletter to share the science behind natural medicines. To support her effort, consider a paid or founding subscription to Nature’s Pharmacy or donation to her lab research.
The Plant Hunter is available in hardcover, paperback, audio, and e-book formats!






This is very cool information Cassandra. Thank you for posting it.
One of the most exciting moments in my years as a research doctoral candidate at Dartmouth Medical School was when I learned that microbes engage in their own survival strategies. Many bacteria, for example produce bacteriocins, positively charged peptides that can kill or inhibit other bacteria. Some bacteriocins, particularly those from Gram-negative bacteria, exhibit structural similarities to eukaryotic defensins, the antimicrobial peptides produced by higher life forms including humans.