Medicinal Plants in Dermatology: Filsuvez (Birch Triterpenes)
Learn about the history, composition, and modern applications of a scientifically proven plant-derived therapy for skin conditions.

This post is part of a series where I break down the topics covered in our recently published review article on plants used in dermatology. Today, we’re covering Filsuvez (Birch Triterpenes).
Israyilova, A., T.V. Peykova, B. Kittleson, P.C. Sprowl, T.O. Mohammed, C.L. Quave. (2025) From Plant to Patient: A Historical Perspective and Review of Selected Medicinal Plants in Dermatology. JID Innovations, 5:1: 100321
Discovery and Composition
Filsuvez, also known as Oleogel-S10, is a wound-healing gel derived from birch bark (Betula pendula and Betula pubescens in the Betulaceae plant family). It contains a betulin-rich triterpene extract, along with refined sunflower oil. Approved by the FDA in December 2023, Filsuvez is used to treat epidermolysis bullosa (EB) based on successful phase 3 trials. Its key ingredients include betulin (72–88%), betulinic acid, lupeol, and other compounds. Birch bark has a long history in European traditional medicine, treating conditions like urinary infections, rheumatism, asthma, and skin issues.
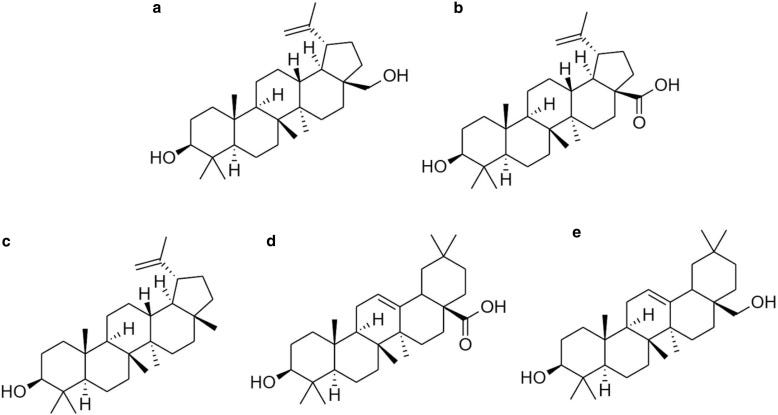
History as a Drug
Betulin, derived from birch bark, was first identified in 1788 and named in 1831 (see above figure for chemical structure). Interest in its medicinal properties surged in 1995 after a study highlighted betulinic acid’s potential against melanoma. Betulin’s ability to stabilize water-in-oil emulsions led to its use in pharmaceuticals like Oleogel-S10, a gel studied extensively for wound healing.
Phase II trials showed promising results: Oleogel-S10 accelerated wound healing in skin graft patients and those with dystrophic epidermolysis bullosa (EB), outperforming control treatments. A larger phase III trial confirmed faster re-epithelialization of donor site wounds, while another demonstrated superior healing for burn wounds compared to Octenilin gel. In the EASE trial, Oleogel-S10 significantly improved wound closure rates in EB patients.
Well-tolerated in all clinical studies, Oleogel-S10 has also shown potential for treating skin conditions like actinic keratoses, highlighting its therapeutic versatility.
Mode of Action
Wound healing happens in three stages. First, the inflammatory phase recruits immune cells to clean the wound. Next, skin cells multiply and migrate to close the wound. Finally, in the longest phase, skin cells mature and remodel to restore the tissue. Birch bark extract enhances this process by stabilizing inflammation mediators like COX-2 and IL-6, promoting keratinocyte migration and accelerating re-epithelialization. It also stimulates keratinocyte differentiation, increasing proteins like involucrin and keratin 10 to strengthen the skin barrier. This extract effectively supports all stages of healing, speeding recovery and restoring healthy skin.
Uses in Dermatology Today
Epidermolysis Bullosa (EB)
Oleogel-S10 has been shown to improve wound healing in patients with epidermolysis bullosa (EB), a rare genetic condition that causes fragile, blistering skin. EB affects about 1 in 17,000 births, with an estimated 500,000 cases worldwide. It is caused by mutations in over 20 genes that affect skin structure, leading to more than 30 subtypes with varying symptoms. According to the Mayo Clinic, symptoms of epidermolysis bullosa include:
Fragile skin that blisters easily, especially on the palms and feet
Nails that are thick or unformed
Blisters inside the mouth and throat
Scalp blistering and hair loss (scarring alopecia)
Skin that looks thin
Tiny pimple-like bumps (milia)
Dental problems, such as tooth decay
Difficulty swallowing
Itchy, painful skin
First approved in 2016 by the European Medicines Agency (EMA) as Episalvan, the birch triterpene gel was relaunched in 2022 as Filsuvez for EB treatment in adults and children over six months old. In 2023, the FDA approved Oleogel-S10 for treating dystrophic and junctional EB, offering hope to those living with this challenging condition.
If interested in taking a deeper dive into this important medication, read my prior post on the clinical findings leading to FDA approval:
Birch extract approved by FDA for rare disease
Epidermolysis bullosa (EB) is a rare genetic disorder. Children born with EB have extremely fragile skin and blisters form on their skin, mucosal tissues, and internal linings of their organs. The underlying cause of the dise…
I hope you enjoyed learning a bit about this important plant-derived medicine!
Yours in health, Dr. Quave
Cassandra L. Quave, Ph.D. is a Guggenheim Fellow, CNN Champion for Change, Fellow of the National Academy of Inventors, recipient of The National Academies Award for Excellence in Science Communication, and award-winning author of The Plant Hunter. Her day job is as professor and herbarium curator at Emory University School of Medicine, where she leads a group of research scientists studying medicinal plants to find new life-saving drugs from nature. She hosts the Foodie Pharmacology podcast and writes the Nature’s Pharmacy newsletter to share the science behind natural medicines. To support her effort, consider a paid or founding subscription to Nature’s Pharmacy or donation to her lab research.
Available in hardcover, paperback, audio, and e-book formats!






